- Home
- Medical devices state registration
- State registration support
Medical device state registration support in Russia
We offer full support of medical devices state registration in Russia.
Your medical devce will be registered by professionals with dozens of
registration certificates in the portfolio and experience in all major areas of medicine:
- Auxiliary and general purpose medical devices
-
Medical products for in vitro diagnostics (IVD)
-
Medical device for manipulation/restoration of tissues/organs
-
Medical devices for plastic surgery, dermatology and cosmetology
-
Orthopedic medical devices
-
Ophthalmic medical devices
-
Radiological medical devices
-
Cardiovascular medical devices
-
Dental medical devices
-
Urological medical devices
-
Physiotherapy medical devices
-
Surgical instruments/systems and related medical devices
We closely cooperate with authorities, federal and regional medical informational-analytic centers and large healthcare institutions, Russian and foreign industrial associations and unions, scientific and expert organizations, as well as leading specialists and experts in the field of medical devices.
At your request the service is available as a whole or at any stage:
1 - Dossier collection / documents elaboration if necessary
2 - Expert review at Roszdravnadzor (stage I)
3 - Clinical data validation
4 - Expert review at Roszdravnadzor (stage II)
Additional services?
- Conformity declaration execution;
- Documentation elaboration consulting;
- Specialized medtech documents tranlsation;
- Documents elaboration of any complexity:
→
device specifications, risk management file, technical file, user manual, etc.,
→
design documentation, technological, etc.
- Measuring equipment type confirmation certificate receiving support.
FOR YOUR INFORMATION:
Registration stages
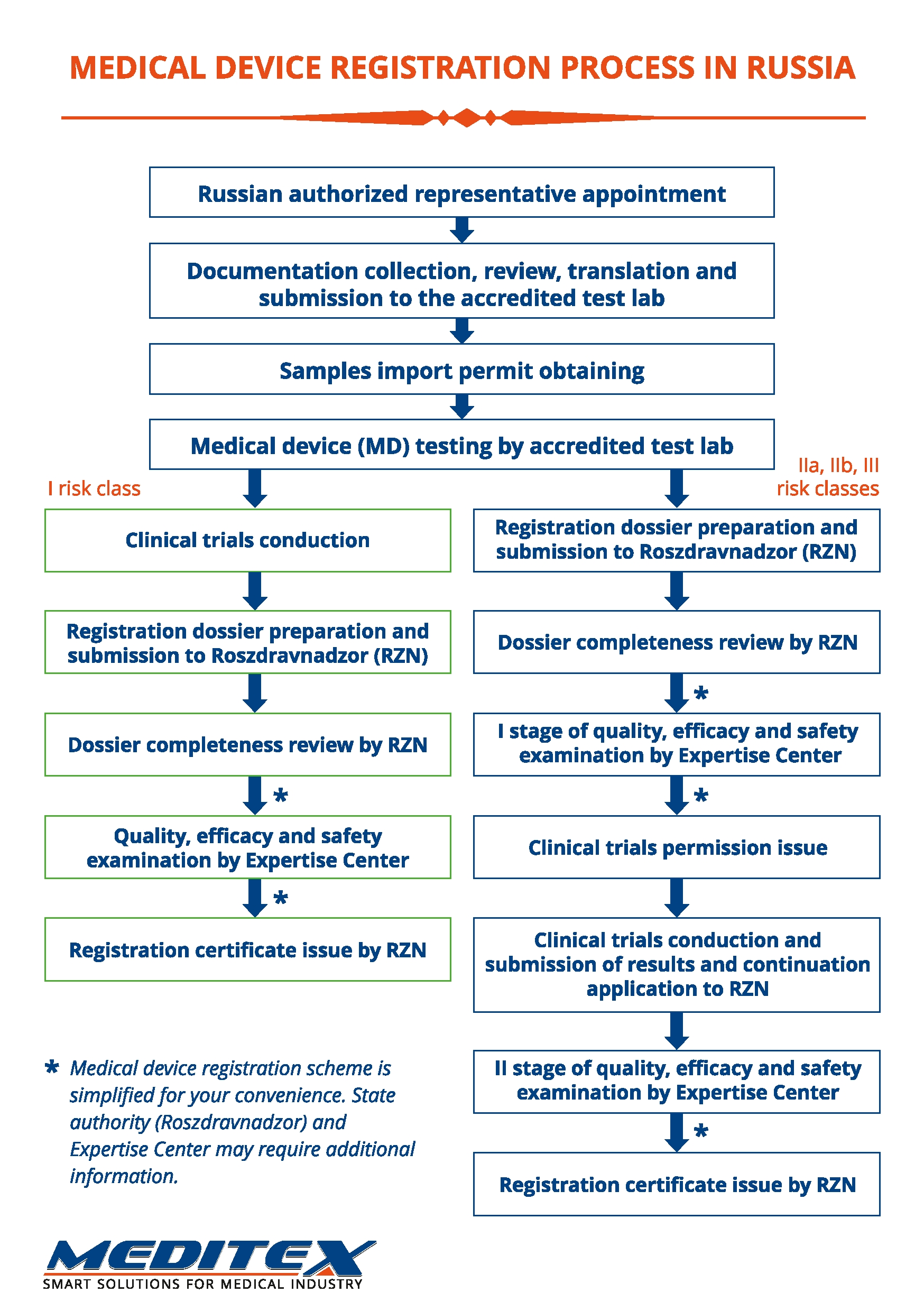
1. Preparation of documentation
1.1. Initial examination of documentation for compliance with the requirements of Roszdravnadzor.
1.2. Documentation amendment according to the requirements of Roszdravnadzor.
1.3. Obtaining permission to import samples (only for goods of foreign production).
1.4. Trials:
1.4.1. Toxicological trials conduction.
1.4.2. Technical trials conduction.
1.5. Acquisition and submission dossier to Roszdravnadzor.
2. Expert review by Roszdravnadzor (I stage)
2.1. Set of registration documents is checked by Roszdravnadzor for completeness and accuracy of the information provided.
2.2. Expert examination of quality, efficacy and safety is conducted by the expert organization in a period not exceeding 20 working days.
2.3. At the end of the examination conclusion is made on possibility (impossibility) of clinical trials conduction (for medical devices of risk class 2A, 2B and 3).
3. Confirmation of clinical data
3.1. Clinical trials conduction.
3.2. Clinical data submission.
4. Expert review by Roszdravnadzor (II stage)
4.1. Clinical data expert examination in a period not exceeding 10 working days.
4.2. At the end of the examination conclusion is made on registration certificate issue.
NOTE: Stages I and II of the expert review for devices of risk class 1 are combined.
Medical device risk classes
In accordance with the order of Ministry of health of 6 June 2012 N 4n medical devices according to potential risk of application are subdivided into four classes. Classes are labeled as follows:
- class 1 - medical devices with a low degree of risk;
- class 2A - medical devices with a medium degree of risk;
- class 2B - medical devices with a heightened degree of risk;
- class 3 - medical devices with a high degree of risk.
Classification of medical devices for in vitro diagnostics:
-
class 1 - medical devices with a low individual risk and low risk to public health;
- class 2A - medical devices with moderate individual risk and/or low risk to public health;
- class 2B - medical devices with high individual risk and/or moderate risk to public health;
- class 3 - medical devices with high individual risk and/or high risk to public health.
State duty amount
In accordance with the risk class state duty amount is determined for examination of quality, efficacy and safety of medical devices:
class 1 - 45 000 RUB;
class 2A - 65 000 RUB;
class 2B - 85 000 RUB;
class 3 - 115 000 RUB.
>> Documents list for MD registration procedure >>
